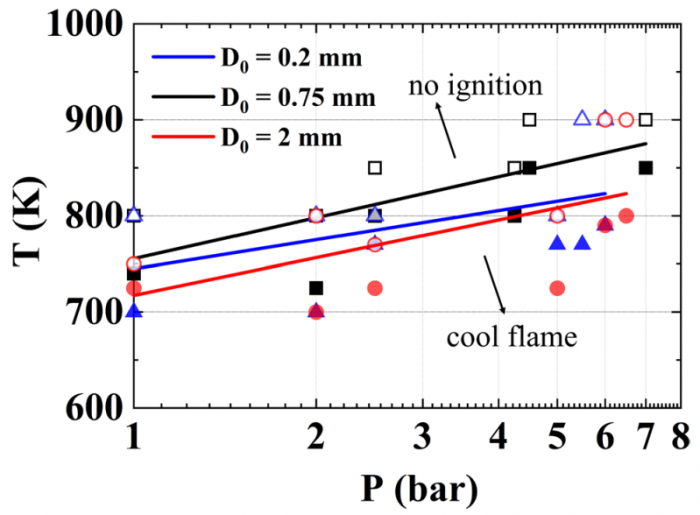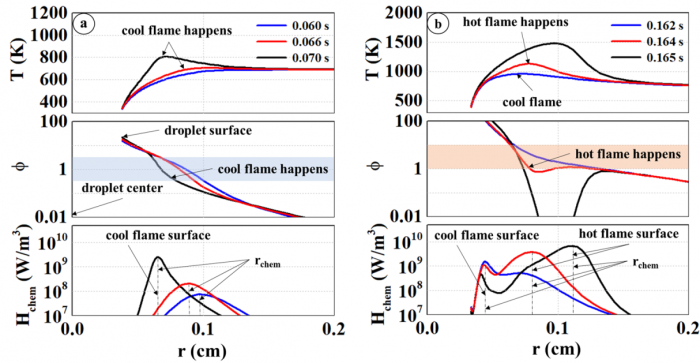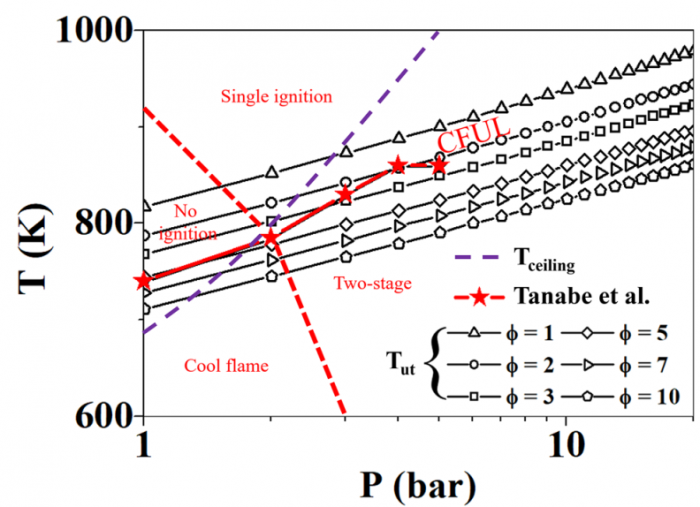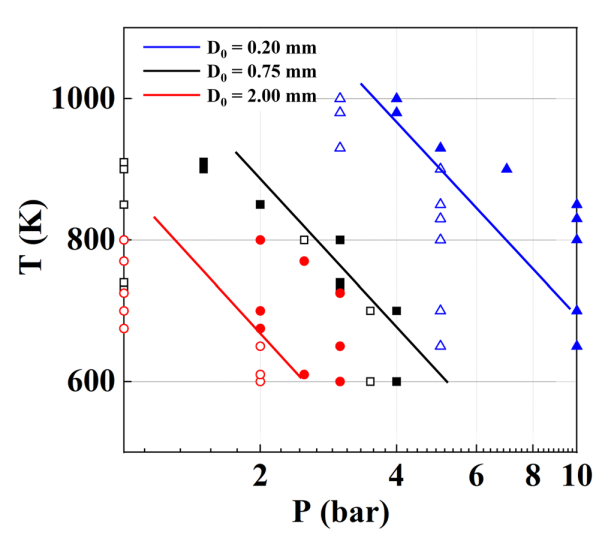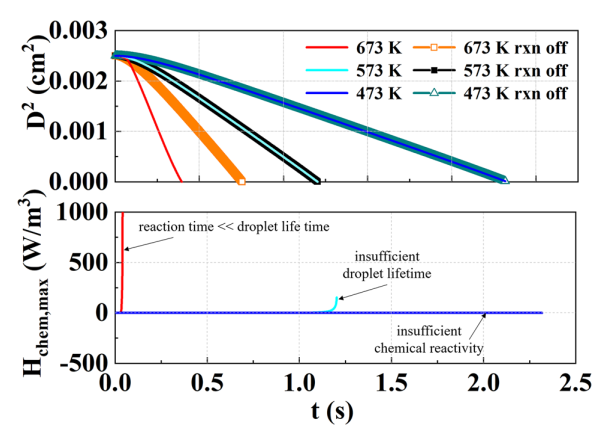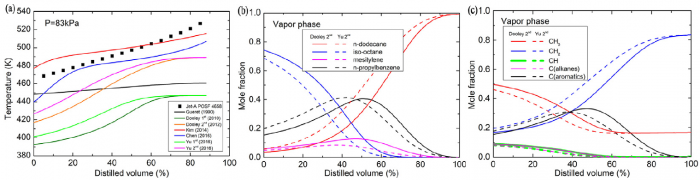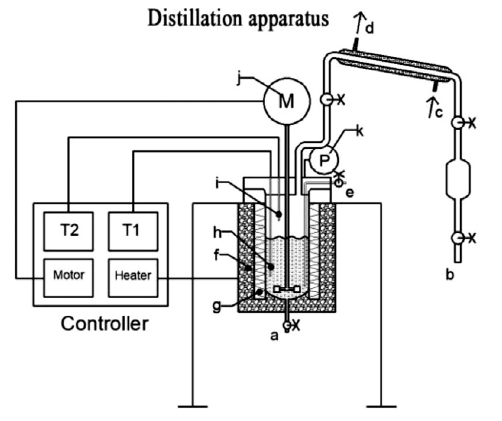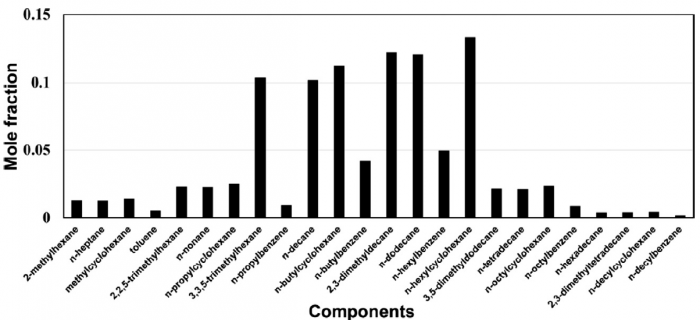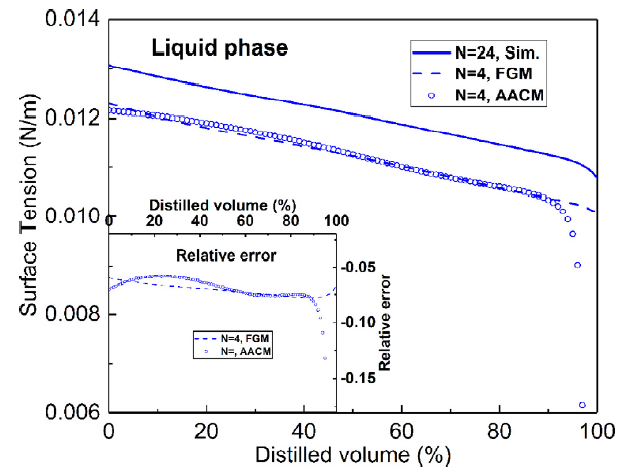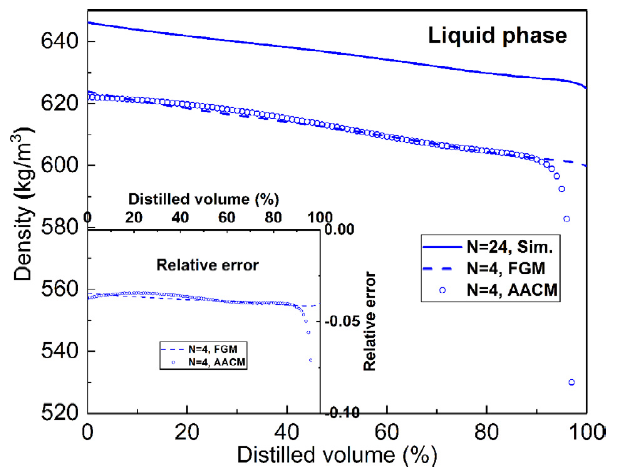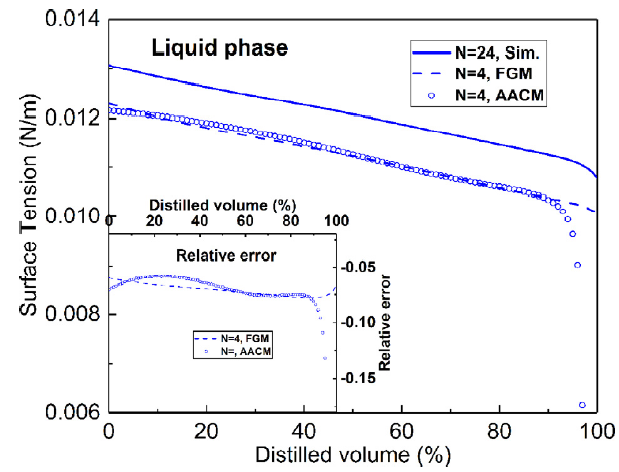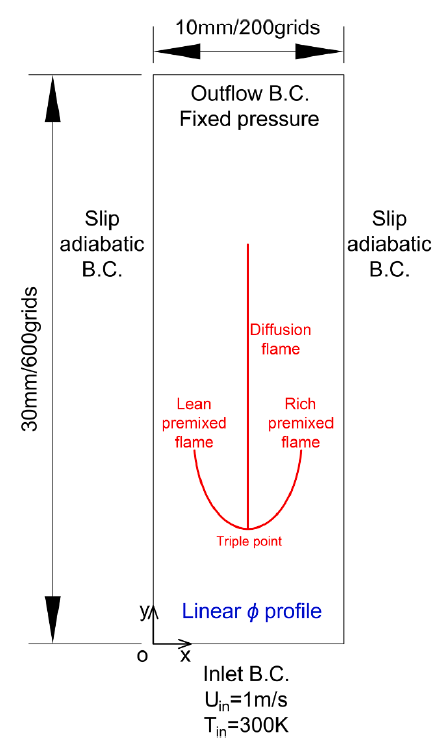
T. Chen, S. Yu, Y.C. Liu, "Effects of pressure on propagation characteristics of methane-air edge flames within two-dimensional mixing layers: A numerical study", Fuel 301 (2021) 120857.
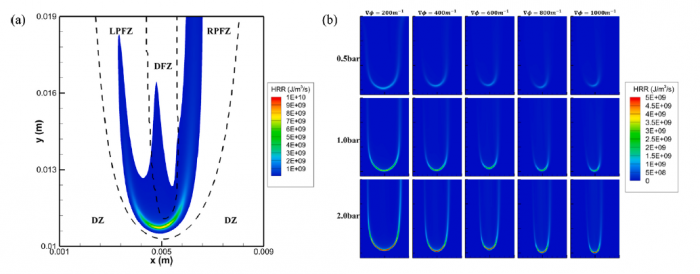
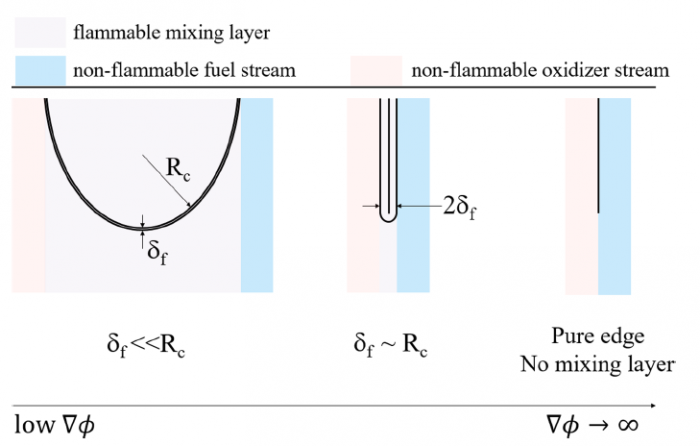
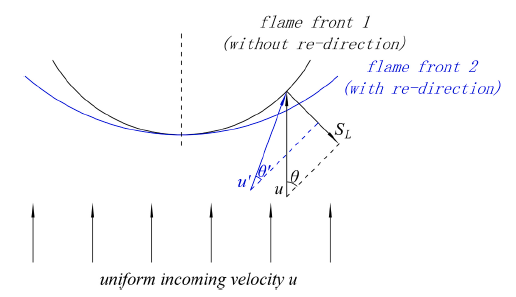
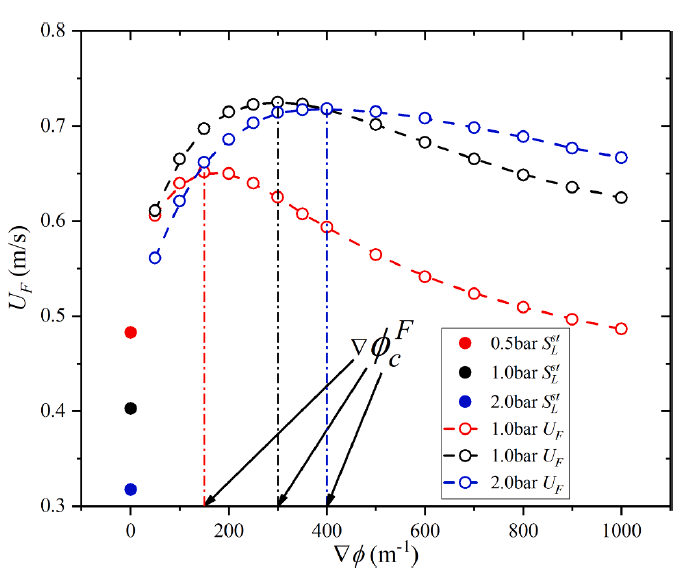
The effects of pressure on propagation of laminar edge flames were numerically investigated using an OpenFOAM-6 platform developed with multicomponent transport properties. With various equivalence ratio gradients (∇ϕ) as inlet conditions, propagation of non-stationary edge flames in two-dimensional mixing layers at three pressures (0.5 bar, 1.0 bar and 2.0 bar) were studied. The heat release rate increases with increasing pressure and decreases with increasing ∇ϕ due to flame curvature and stretch. The linearity between flame curvature K and ∇ϕ is revealed. The K and slope of this linearity are both larger under high pressure. Flame stretch is dominated by flow strain and flame curvature rather than unsteady flame motion. With increasing ∇ϕ and pressure, stretch rate κ shows increasing feature due to larger curvature and stronger flow strain. The obvious negative linear dependence of local flame speed Sd on κ is revealed under three pressures. The range of Karlovitz number under three pressures are 0.2∼ 1.4, indicating that edge flames are weakly stretched and the linear correlation between Sd and κ could be explained by weakly stretched flame theory. Compared with positive dependence of mass diffusion term Sd d on K, the dominating negative dependence of reaction term Sr d on K leads to negative correlation of Sd with K. Global flame speed UF shows non-monotonic feature with increasing ∇ϕ under three pressures, which is mainly due to non-monotonic upstream velocity reduction Udrop. Flame stretch is important for the shift from critical gradient ∇ϕdc (for maximum Udrop) to smaller ∇ϕFc (for maximum UF) and stronger decreasing feature of UF thanUdrop under large ∇ϕ.
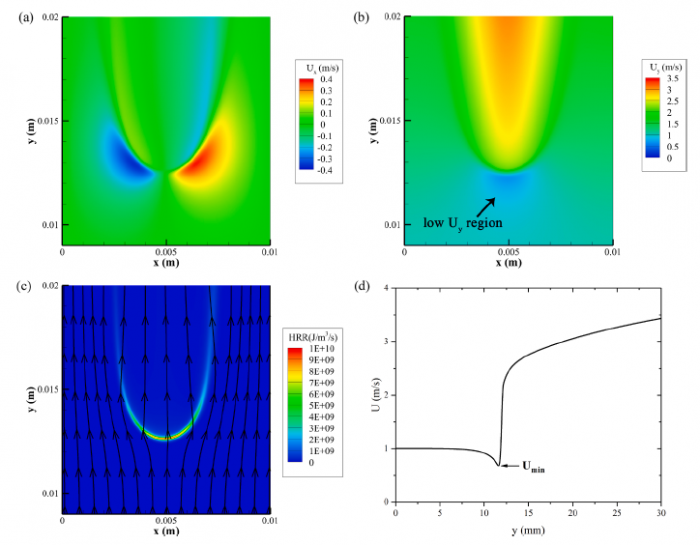
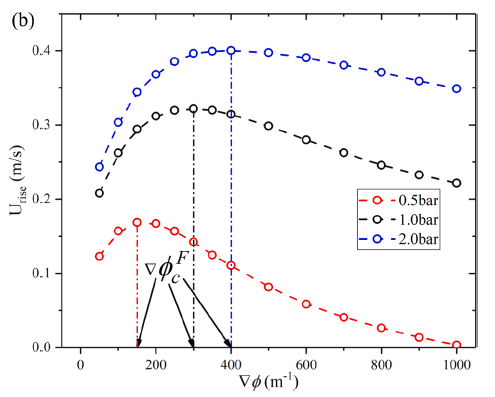
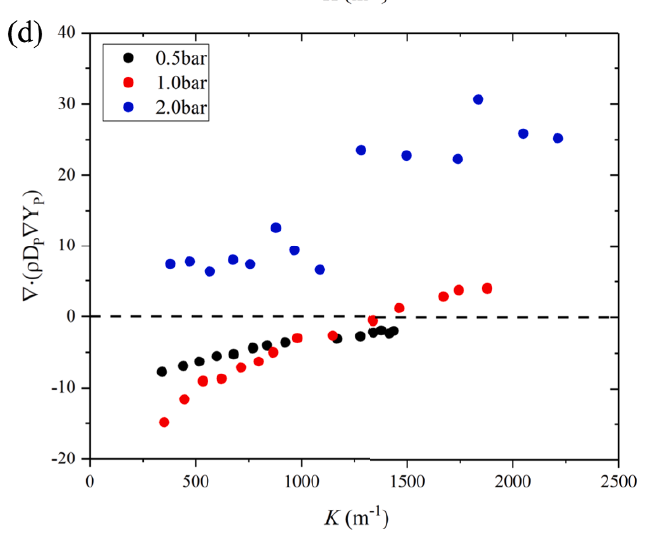
----------------------------------------------------------------------------------------------------------
T. Chen, S. Yu, Y.C. Liu, "Soret effects on the diffusion-chemistry interaction of hydrogen-air edge flames propagating in transverse gradient evolving mixing layers", Fuel 315 (2022) 123014.
The effects of Soret diffusion (SD) on the hydrogen-air edge ame propagation and the diffusion-chemistry interaction are investigated through simulation facilitated by the numerical code MultiDiffFOAM. The edge ames in this study gradually develop from a ame kernel into a tri-brachial structure in a hydrogen-air mixing layer that temporally evolves due to transverse reactant concentration gradient. We demonstrate that the responses of ame displacement speed Sd to ame curvature K, stretch rate and scalar dissipation rate are distinctly influenced by SD. For the linear Sd-K and Sd-X correlations, SD would result in a smaller Markstein length. Moreover, SD is shown to lead to shifting of the Sd-X curve towards the regime with larger X. Compared with the weak influences of SD on the tangential diffusion component Sd;t and normal diffusion component Sd;n, the chemical reaction component Sd;r is significantly weakened by SD. The important chemical reactions for edge ame propagation are identified based on sensitivity analysis and their rates are found to be smaller when SD is considered. For the local composition at the ame marker, the mass fraction of H2 is slightly larger and that of H is obviously smaller when SD is considered. The SD flux of H2 jSD H2 and that of H jSDH are both coupled with the driving force grad(lnT) along the mixture fraction coordinate. However, the jSD H2 is mainly concentrated on the unburnt side while the jSD H is on the burnt side. The analyses on decomposed uxes of H2 and H along the flame normal direction further suggest that SD would enhance the H2 mass diusion but weaken the H mass diffusion. Such opposite effects stem from the distribution features that H2 is mainly on the unburnt side while H on the burnt side.
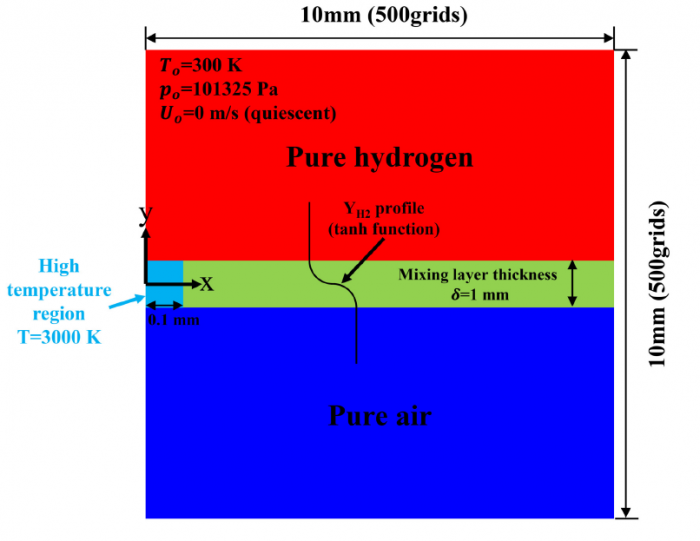
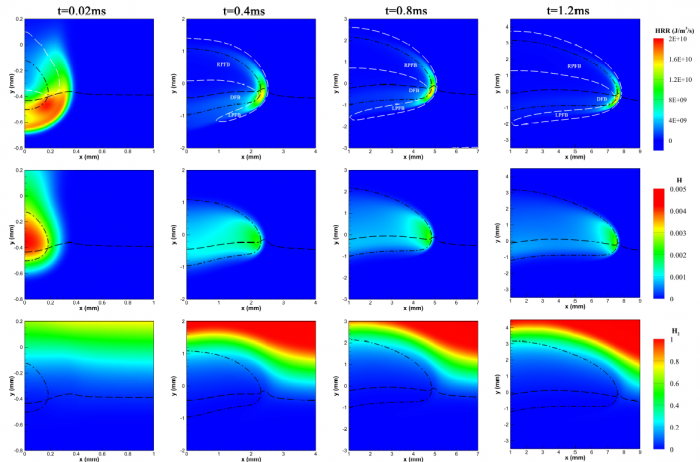
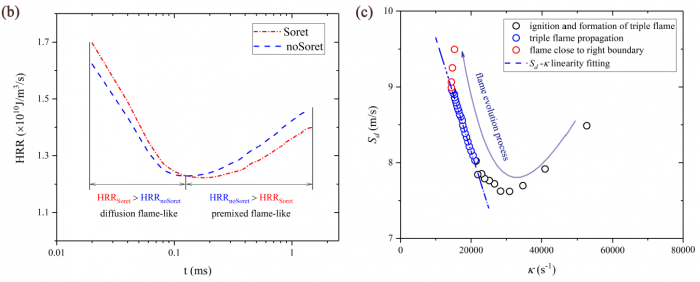
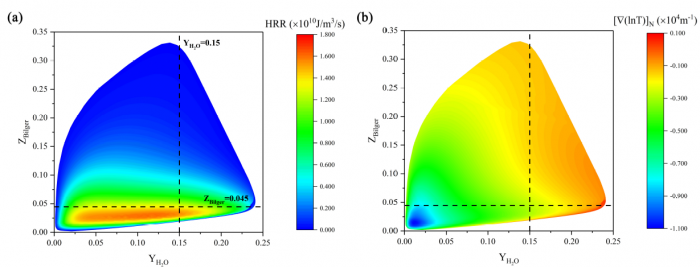
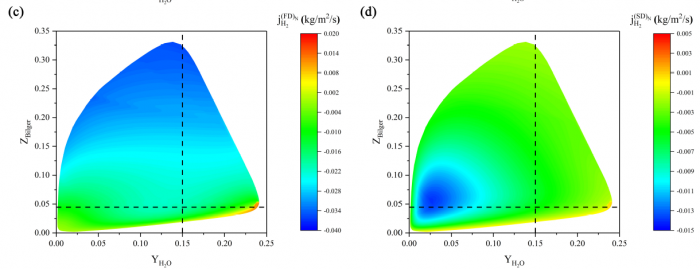
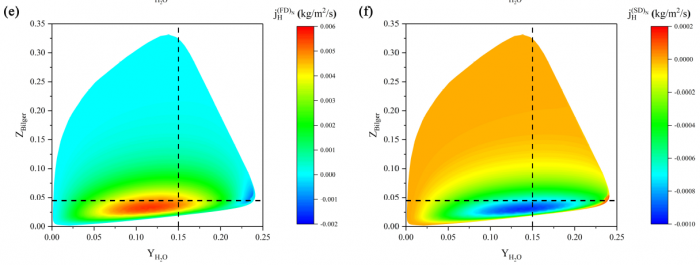
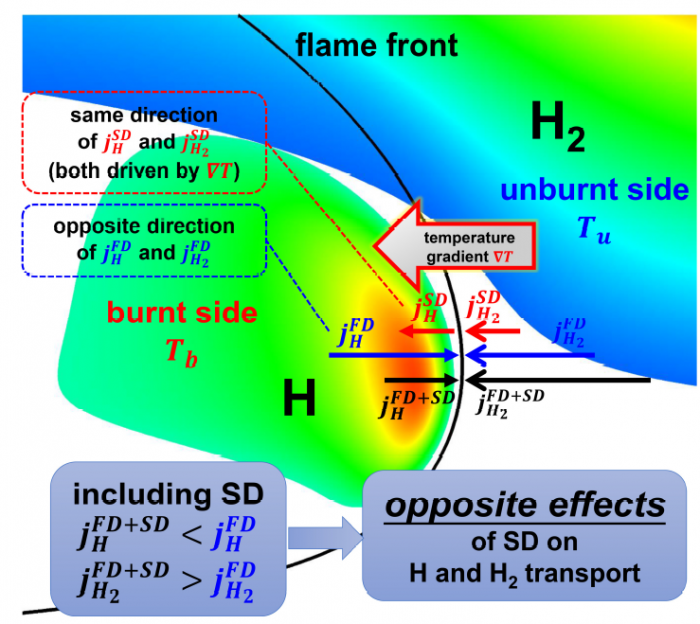
----------------------------------------------------------------------------------------------------------
T. Chen, S. Yu, Y.C. Liu, "Flow strain and curvature Markstein numbers of edge flame in the counterflow configuration", International Journal of Hydrogen Energy (2022) in press.
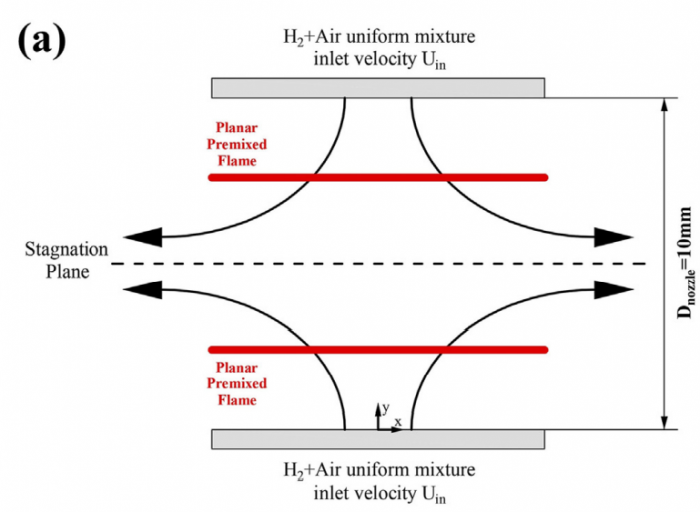
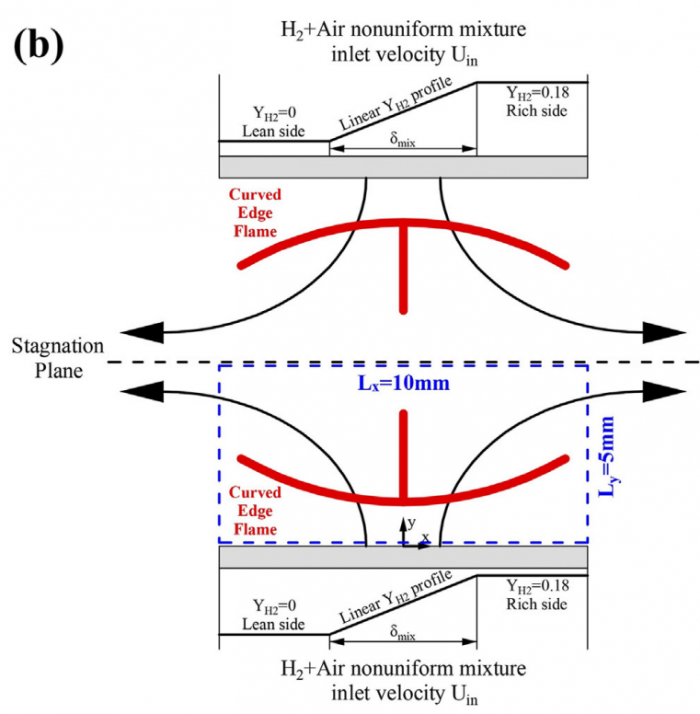
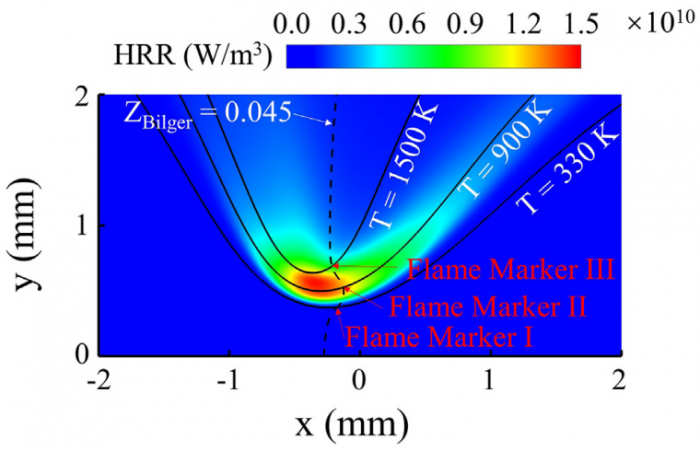
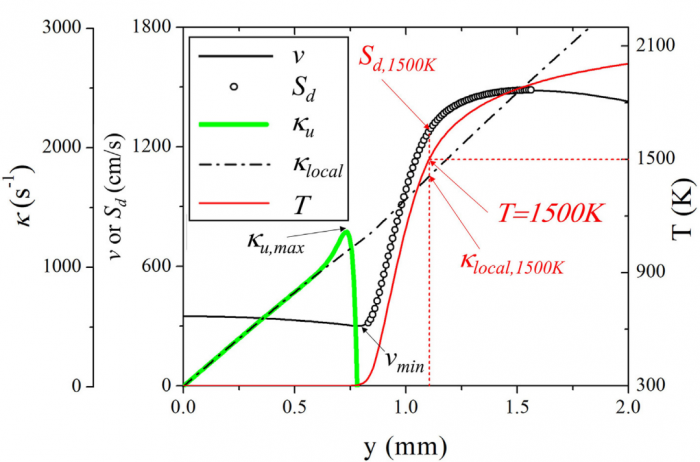
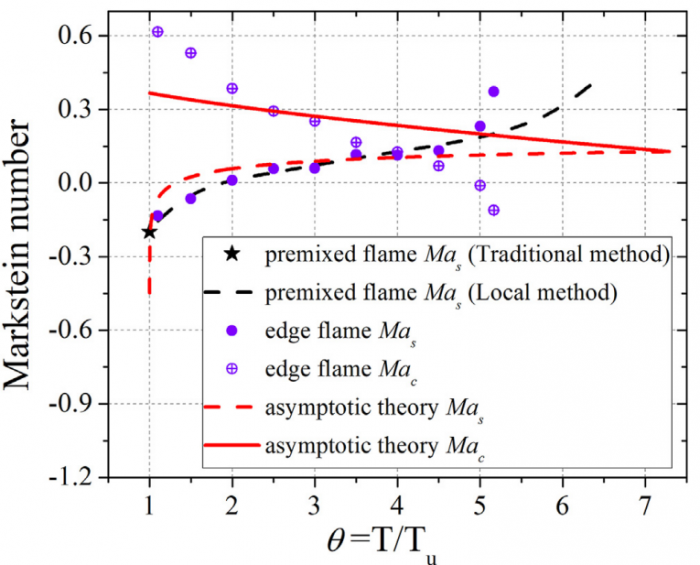
Based on the counterflow configuration, the Markstein numbers of one-dimensional pre-mixed flame and two-dimensional edge flame of hydrogen-air system are numerically studied and compared with each other. By varying the inlet velocity and mixing layer thickness, the proportional relationship between flame stretch caused by flow strain (Ka s) and by flame curvature (Ka c) disappears, and therefore two corresponding Markstein numbers Ma s and Ma c at different flame markers (or iso-contours of temperature) within flame front could be obtained through linear fitting between normalized flame displacement speed S * d and bivariate (Ka s ; Ka c). With the flame marker shifting from unburnt to burnt side, Ma c profile of edge flame exhibits a decreasing trend while Ma s profiles of premixed flame and edge flame both display an increasing trend with its sign changing from negative to positive. Moreover, a good agreement of Ma s profiles between two kinds of flames is observed while the discrepancy begins to appear when normalized temperature q !5, which should be attributed to the shift of flame marker from premixed branch into diffusion branch rather than the decrease of heat release rate. The comparison between simulation and theory reveals differences of Ma s values except for 2.5 < q < 4 and those of Ma c values except for q ¼ 2.5, which might be accounted for a moderate Zel'dovich number Ze, i.e. 3.9, based on detailed hydrogen-air chemical mechanism. Therefore, the "larger activation energy" assumed in asymptotic theory may not be valid for hydrogen-air flames whose equivalence ratio is 1.6 in this study.