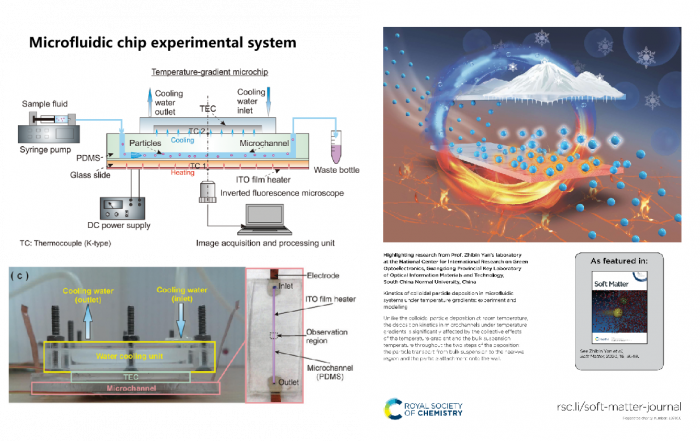
Energy conversion:Photothermal effect of metal plasma structure on the surface of nanoparticles
In this work, we study the effects of the morphology of gold clusters on the surface of SiO2@Au core–shell nanoparticles on their photothermal conversion performance by changing the amount of gold salt, the pH value of the growth solution, and the volume and concentration of the reducing agent used in the chemical synthesis of SiO2@Au.
The morphology of gold clusters on the surface of silica core were characterized by calculating the equivalent circular diameter of gold clusters (Dave) and the average distance among gold clusters (Lave) on the silica core surface based on the measurements on TEM image of SiO2@Au using the software ImageJ. Based on the measured temperature rise of the SiO2@Au droplets and the calculated Dave and Lave of the gold cluster, the results show the temperature rise of the SiO2@Au dispersions depends on the size of gold clusters when the ratio of gold clusters' equivalent diameter to average distance among the clusters (Lave/Dave) is greater than 1.3. On the contrary, the strong coupling effect between gold clusters play an important role in photothermal effect when the ratio is less than 1.3.
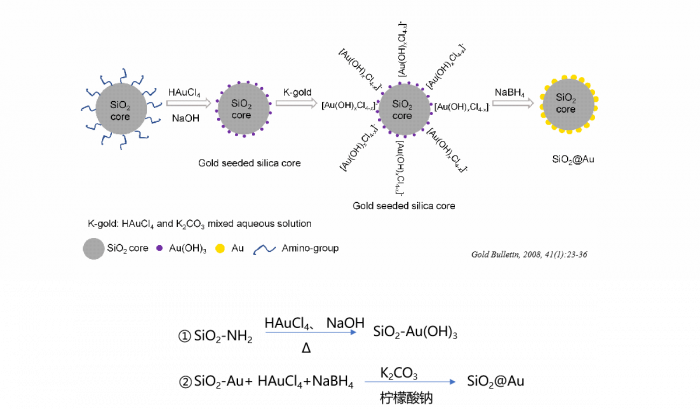
Figure1. Synthetic procedures of the SiO2@Au core–shell nanoparticles. We study the effects of the morphology of gold clusters on the surface of SiO2@Au core–shell nanoparticles on their photothermal conversion performance by changing the amount of gold salt, the pH value of the growth solution, and the volume and concentration of the reducing agent used in the chemical synthesis of SiO2@Au.
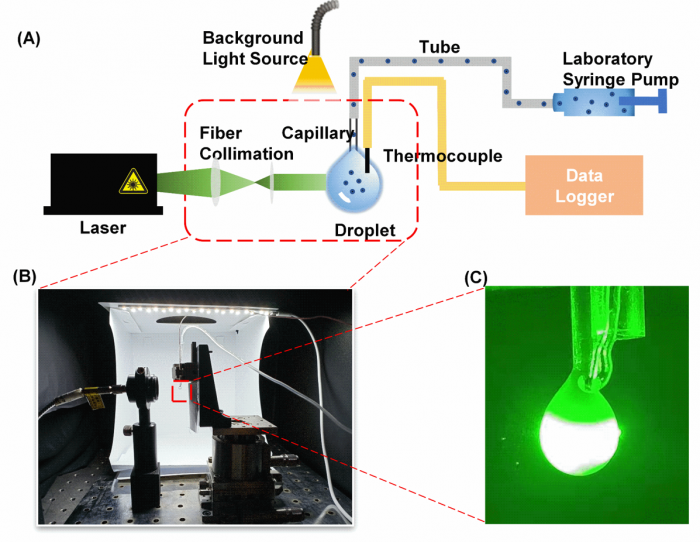
Figure2. Photothermal effect testing device: (A) Schematic of the experimental setup for characterizing the photothermal performance. (B) Photo of the experimental setup. (C) An image showing the laser passing through the droplet.
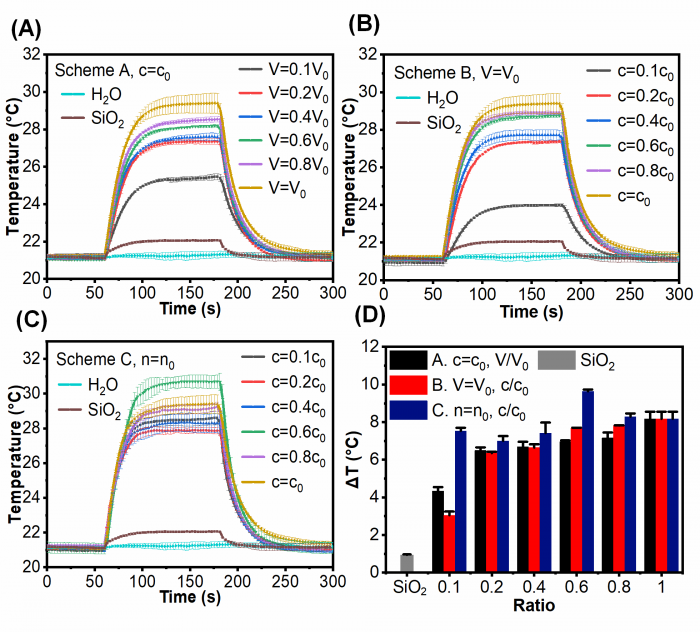
Figure3. Temperature evolutions of Scheme A (A), Scheme B (B) and Scheme C (C) under laser irradiation. The power of the laser was fixed at 53.6 mW. (D) Temperature rise after 120 seconds laser irradiation of Schemes A, B and C, and the abscissa represents the ratio of c/c0 or V/ V0 (volume ratio of K-gold to seed: 80 : 1 and pH ¼ 10.31, V0 ¼ 4 mL and c0 ¼ 6.6 mM of NaBH4).

Figure4. The influence of NaBH4 participating in metallization of gold-seeded silica nanoparticles. (A) The TEM images of SiO2@Au with different volume of NaBH4 at V ¼ 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 V0 (i–vi) while keeping concentration constant, and (B) the corresponding UV-Vis absorption spectra for Scheme A. (C) The TEM images of SiO2@Au with different concentration of NaBH4 at c ¼ 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 c0 (i–vi) while keeping volume constant and (D) the corresponding UV-Vis absorption spectra for Scheme B. (E) The TEM images of SiO2@Au under different concentration of NaBH4 at c ¼ 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 c0 (i–vi) while keeping amount of substance constant and (F) the corresponding UV-Vis absorption spectra for Scheme C. (volume ratio of K-gold to seed: 80 : 1, and pH ¼ 10.31, V0 ¼ 4 mL and c0 ¼ 6.6 mM of NaBH4).
Articles: