分享到
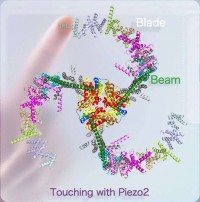
Tethering Piezo channels to the actin cytoskeleton for mechanogating via the E-cadherin-β-catenin mechanotransduction complex
2020
其它
- Cold Spring Harbor Laboratory
- DOI: 10.1101/2020.05.12.092148