分享到
Theoretical Exploration of Single-Layer Tl2O as a Catalyst in Lithium–Oxygen Battery Cathodes
2020
期刊
The Journal of Physical Chemistry C
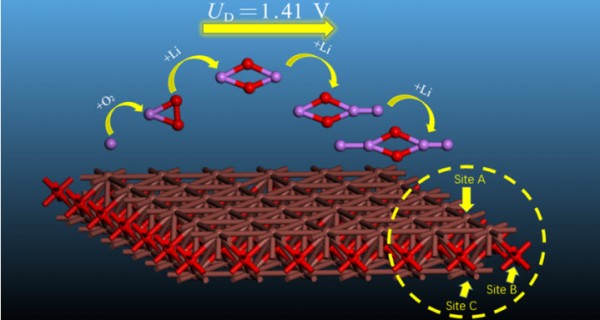
- 卷 124
- 期 17
- 页码 9089-9098
- American Chemical Society (ACS)
- ISSN: 1932-7447
- DOI: 10.1021/acs.jpcc.9b09665



